By Tamzyn Murphy (RD, MSc)
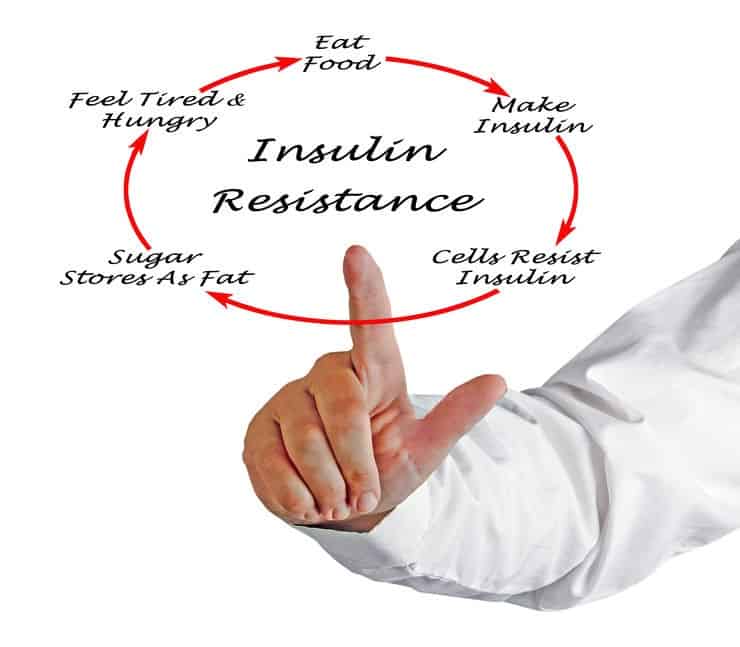
Does going low carb make you insulin resistant? It does indeed! “But how can that be?!” you may ask. Especially considering that the evidence in support of LCHF for insulin resistant conditions (e.g. metabolic syndrome [1-3], Polycystic Ovarian syndrome [4, 5], prediabetes [6, 7], and, last but certainly not least, Type 2 Diabetes [8-11]) is overwhelmingly in favour of LCHF diets: LCHF sees improvements in Hba1c and insulin concentrations, triglyceride to HDL ratio, as well as reductions in hypoglycaemic medication and body weight [8, 9, 11] – all clear signs of improvements in insulin resistance, health and prognosis. Yet, when people who usually restrict carbs suddenly indulge in high-carb foods – even if they were never insulin resistant before – signs and symptoms of insulin resistance abound. Their blood glucose is clearly not being taken up and used as it should. In response to a carbohydrate load, Low Carbers’ blood glucose sky-rockets and they experience signs and symptoms of hyperglycaemia followed by reactive hypoglycaemia, including nausea, shakiness, light-headedness and sweating. In keeping with this, rodent studies indicate that high fat diets increase insulin resistance [12]. Interestingly, the same thing happens in starvation and fasting by cells downregulating cells’ “machinery” required to process carbs [13, 14]. Carbohydrate restriction, fasting and starvation are all times when you’d typically be losing weight, and if anything, expect to become less insulin resistant (i.e. further way from getting type 2 diabetes). So, what could be behind this low carb/fasting-insulin resistance paradox?
The dissonance between the beneficial metabolic effects seen in insulin resistant conditions on LCHF, and worsening insulin resistance observed on LCHF, may be explained by differences between two insulin resistant states with very different origins and implications:
1.) pathological insulin resistance – a chronic and progressive insulin resistance, caused by excessive circulating glucose and thus, insulin, and associated with the development of type 2 diabetes; and
2.) physiological insulin resistance – a healthy, adaptive, transitory (for as long as carbohydrate restriction continues), and reversible state of insulin resistance, which develops in response to carbohydrate restriction (e.g. a low carb or ketogenic diet or fasted state) [15].
There are likely two reasons for this adaptive physiologic insulin resistance during times of carbohydrate restriction and fasting:
- Changing from being a carb-burner to a fat-burner. The body will always take up and use glucose before other fuels when there’s a lot of glucose around. This is because leaving glucose in the bloodstream results in high blood glucose levels, which are dangerous (think Type 2 Diabetes). However, when you eat a low carb (aka. low glucose) diet, blood glucose levels are reduced and nicely controlled. So, the urgent need for cells to get glucose out of the blood is reduced. Insulin levels also go down, freeing up fat stores to breakdown fat to be used as fuel by cells. Usually this low carb diet is also relatively high in fat (as you have to get your calories from somewhere and you can only eat so much protein). So, between the mobilised fat from storage and extra dietary fat coming in, there’s more fat as fuel available for burning. In response to this switch in fuel availability from carbs (glucose) to fat, cells do an overhaul of their ‘machinery (primarily transport, signalling, and utilisation proteins) from carb-centric to fat-centric. In other words, they become fat-burners, rather than carb-burners. To do this, cells reduce the ‘machinery’ required to use carbs (glucose) (such as proteins used in insulin signalling and glucose uptake and processing) and increase the machinery required to process fats [13]. In other words cells adapt to process the fuel that’s available.
- Ensuring peripheral insulin resistance during times of glucose scarcity ensures that the brain will always have the glucose it needs [13]. Most of the body’s tissues can run off fat. However, some, most importantly nervous/brain tissue, can only use glucose or ketones (which are made from fat) as a fuel. Ketone bodies provide the majority of the brain’s fuel requirements in the context of a ketogenic diet [16], and during fasting or starvation [17]. However, the brain still needs some glucose. An adaptive peripheral insulin resistance ensures that glucose isn’t wasted on the muscles that don’t need it, but can rather be used by the brain that does.
Implications
There are two main implications of this physiological insulin resistance if you’re following a LCHF diet:
- Cheating: Eating a carbohydrate load when in a fat-adapted/ketogenic carbohydrate-restricted state, may result in hyper and reactive-hypo- glycaemia or a milder form of these. This is one of the main reasons why ‘cheating’ on high carb foods is not an option when following a low carbohydrate high fat (LCHF) diet.
- Oral glucose tolerance test (OGTT): This test is the gold standard for diagnosis type 2 diabetes. It entails the patient drinking a lot of glucose (75-100 g, which is more than a low carber gets in 24 – 72 hours); then testing blood glucose levels over the next 2 hours to see how well their cells can take that glucose up out of the bloodstream, thereby restoring normal blood glucose levels. As you can imagine, a metabolic state that’s adapted to using fat rather than carbs (glucose), is ill equipped to process this glucose load. The result may be a false positive for pre- or even full-blown diabetes. As de Oliveira Caminhotto and Lima (2013) succinctly state, “it should be common in clinical practice that patients submitted to oral glucose tolerance tests not be under severe carbohydrate restriction, since this could alter the response to the glucose overload.” [15]
So, if you low carb and feel sick after eating carbs or if you’ve been diagnosed with “diabetes”; based on an OGTT don’t panic! Realise that physiological insulin resistance is likely behind it – not a metabolically unhealthy and pathological state. If you really wanted to, you could train your cells back to insulin sensitivity in a matter of days by reintroducing carbs to your diet [15]… but then you’d lose all the benefits of low carbing that occur in the face of this physiological adaptive insulin resistance. The choice is yours.
| Insulin 101. Insulin is released by the pancreas in response to rising blood glucose concentrations. Insulin acts like a key, unlocking the locks on the cells’ ‘doors’, thereby allowing glucose to leave the bloodstream and enter the cells. This is really important to prevent dangerously high blood glucose. It also provides cells with glucose to burn as fuel. Insulin has other jobs too. One of them is as the master fat-storage regulator. It signals to fat stores to open their entry doors and close their exit doors, thereby allowing fuel in and fat to be built, but none to breakdown or be released into the bloodstream to be used as fuel by other body parts. |
References
- Reaven, G.M., Do high carbohydrate diets prevent the development or attenuate the manifestations (or both) of syndrome X? A viewpoint strongly against. Current opinion in lipidology, 1997. 8(1): p. 23-27.
- Reaven, G.M., The insulin resistance syndrome: definition and dietary approaches to treatment. Annu. Rev. Nutr., 2005. 25: p. 391-406.
- Gershuni, V.M., S.L. Yan, and V. Medici, Nutritional ketosis for weight management and reversal of metabolic syndrome. Current nutrition reports, 2018. 7(3): p. 97-106.
- McGrice, M. and J. Porter, The effect of low carbohydrate diets on fertility hormones and outcomes in overweight and obese women: a systematic review. Nutrients, 2017. 9(3): p. 204.
- Mavropoulos, J.C., et al., The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: A pilot study. Nutrition & Metabolism, 2005. 2(1): p. 35.
- Saslow, L.R., et al., A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PloS one, 2014. 9(4): p. e91027.
- Spritzler, F., A low-carbohydrate, whole-foods approach to managing diabetes and prediabetes. Diabetes Spectrum, 2012. 25(4): p. 238-243.
- Huntriss, R., M. Campbell, and C. Bedwell, The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. European journal of clinical nutrition, 2017: p. 1.
- Meng, Y., et al., Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: A systematic review and meta-analysis of randomized controlled trials. diabetes research and clinical practice, 2017. 131: p. 124-131.
- Cucuzzella, M., et al., A clinician’s guide to inpatient low carbohydrate diets for remission of type 2 diabetes: toward a standard of care protocol. Diabetes Management, 2019. 9(1): p. 7-19.
- Hallberg, S.J., et al., Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Therapy, 2018. 9(2): p. 583-612.
- Bielohuby, M., et al., Impaired glucose tolerance in rats fed low-carbohydrate, high-fat diets. Am J Physiol Endocrinol Metab, 2013. 305(9): p. E1059-70.
- Soeters, M.R. and P.B. Soeters, The evolutionary benefit of insulin resistance. Clinical nutrition, 2012. 31(6): p. 1002-1007.
- Tzagournis, M. and T.G. Skillman, Glucose intolerance mechanism after starvation. Metabolism, 1970. 19(2): p. 170-178.
- de Oliveira Caminhotto, R. and F.B. Lima, Impaired glucose tolerance in low-carbohydrate diet: maybe only a physiological state. American Journal of Physiology-Endocrinology and Metabolism, 2013. 305(12): p. E1521-E1521.
- Westman, E.C., et al., A review of low-carbohydrate ketogenic diets. Current atherosclerosis reports, 2003. 5(6): p. 476-483.
- Owen, O., et al., Brain metabolism during fasting. The Journal of clinical investigation, 1967. 46(10): p. 1589-1595.
